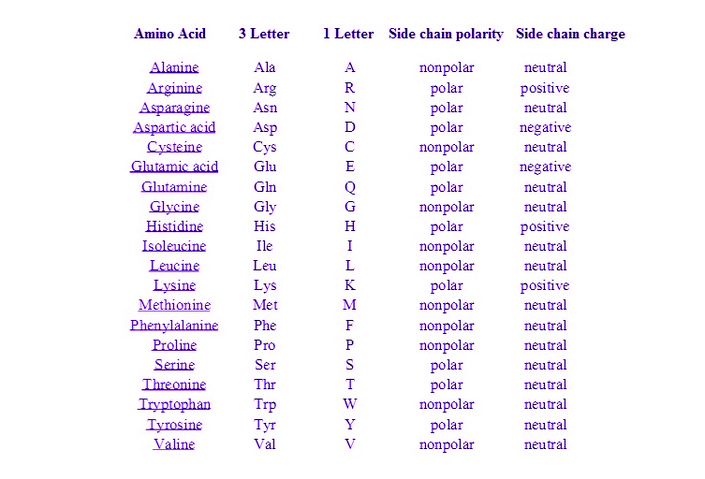
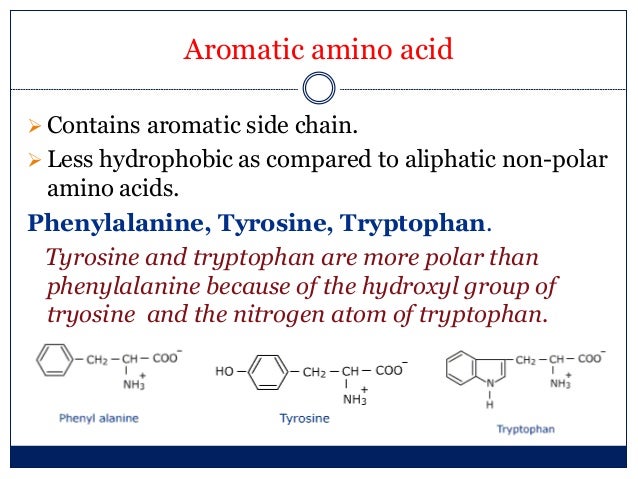
Aromatic Amino Acids Examples

Examples for such amino acids include.
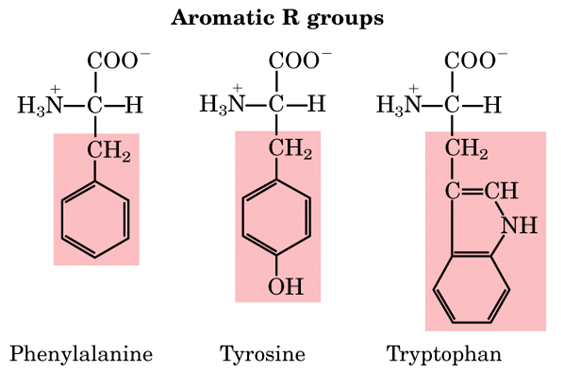
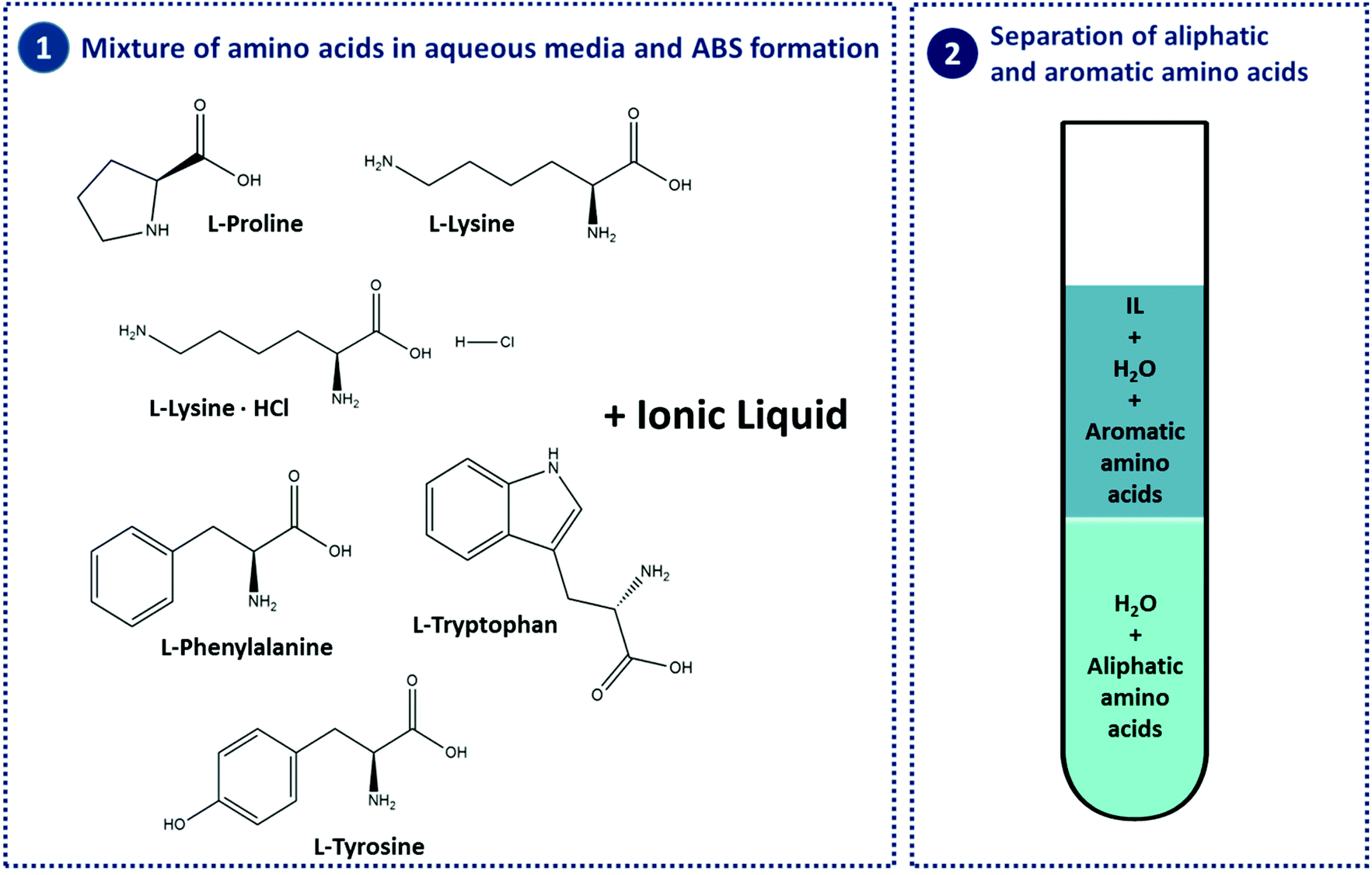
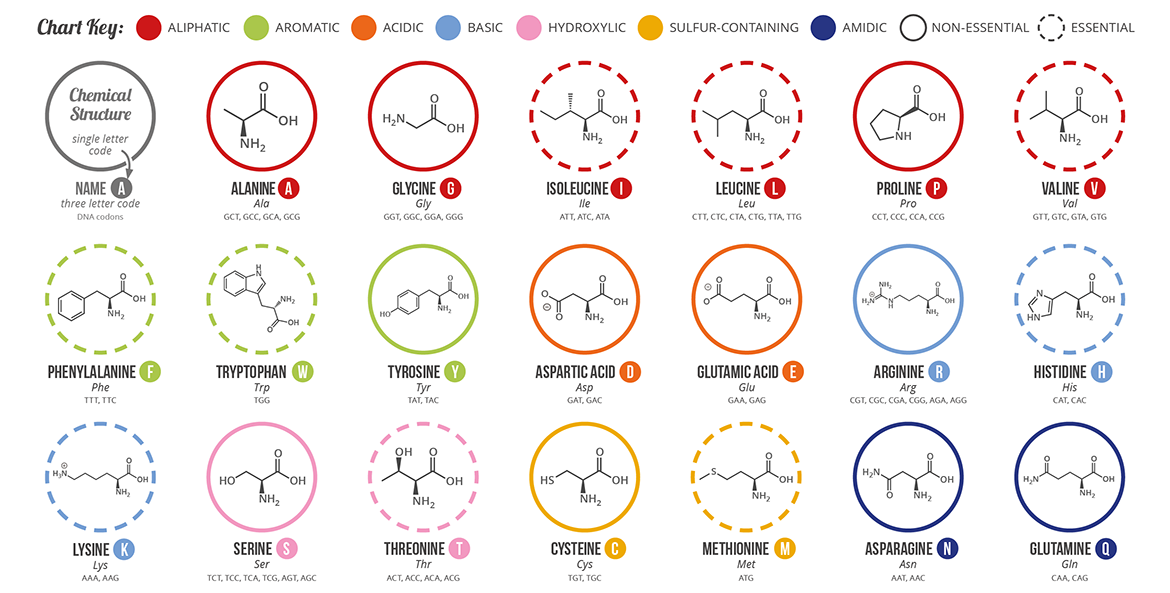
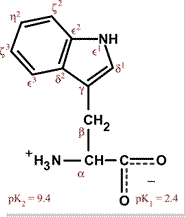
Aromatic amino acids examples. Proline pro or pyrrolidine 2 carboxylic acid and its 4 hydroxylated derivative hydroxyproline contain a heterocycle having 4 carbon atoms and one nitrogen atom. See phenylalanine structure in the above diagram. Each of the 20 most common amino acids has its specific chemical characteristics and its unique role in protein structure and function. Phenylalanine tryptophan and tyrosine however in addition to being aromatic tyrosine can be classified as a polar amino acid.
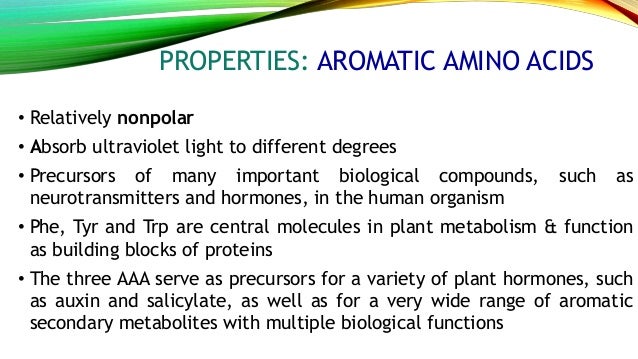
Aromatic carboxylic acids show not only the acidity and other reactions expected of carboxylic acids as an acid benzoic acid is slightly stronger than acetic acid but similar to other aromatic compounds also undergo electrophilic substitution reactions. Least hydrophobic very hydrophobic. A lack of this could lead to depression and a reduced sense of pain. Tyrosine is the only one of the aromatic amino acids with an ionizable side chain.
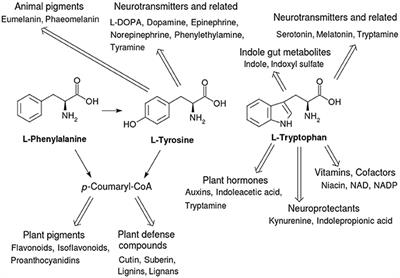
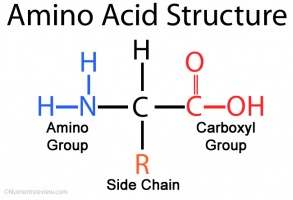
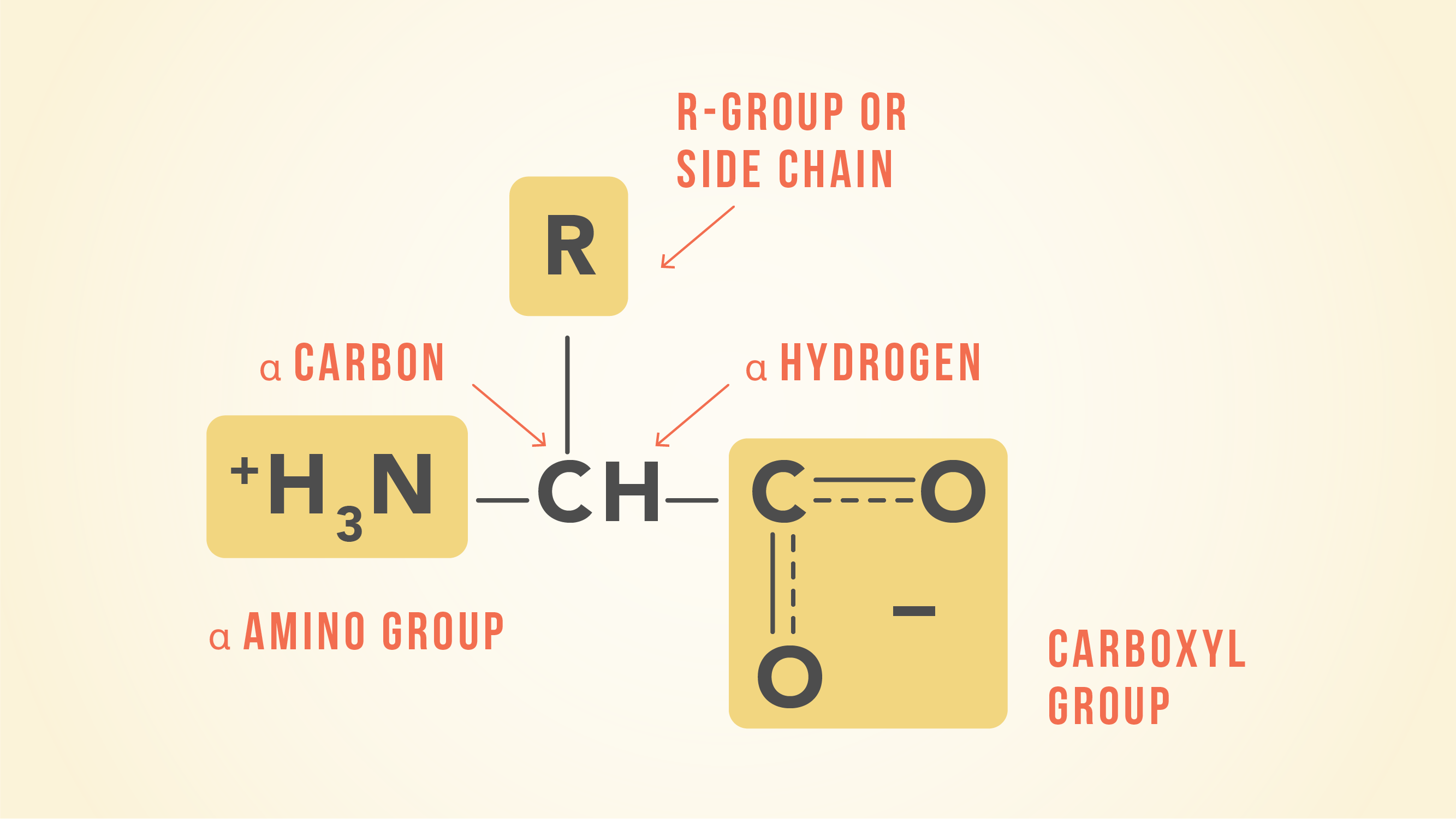
Many amino acids are used to synthesize other molecules for example. The latter is also known as the happiness hormone. The conjunction of a carbonyl and a hydroxyl group forms a functional group known as a carboxyl group. 280 nm by proteins.
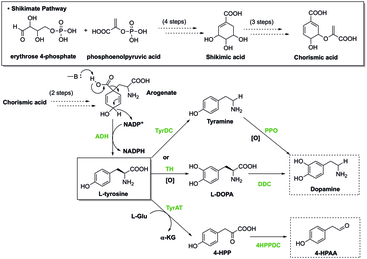
Aromatic rings are the ring like carbon molecules that are double bond and unsaturated in nature. Read more on this topic. The first reaction of this synthesis is catalyzed by deoxyalabino hepturose phosphate synthase dahps where this is an important regulatory enzyme. Furthermore tryptophane is regarded as a basic product for the hormones melatonin and serotonin.
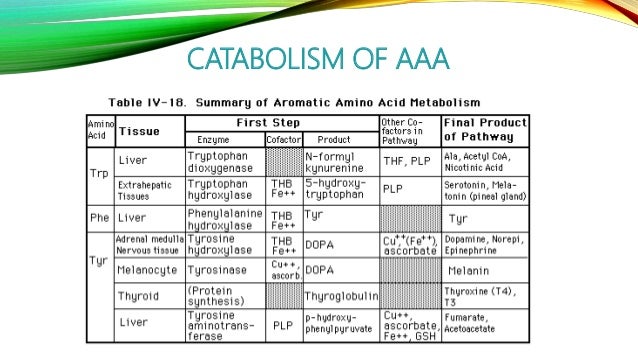
An aromatic amino acid aaa is an amino acid that includes an aromatic ring. The aromatic tryptophan is an essential amino acid and a precursor of niacin which is very important for our energy metabolism. Aromatic amino acids such as tryptophane trp phenylalanine phe and tyrosine tyr are formed from e4p in the pp pathway and pep in the glycolysis. Tyrosine is one of three hydroxyl containing amino acids.
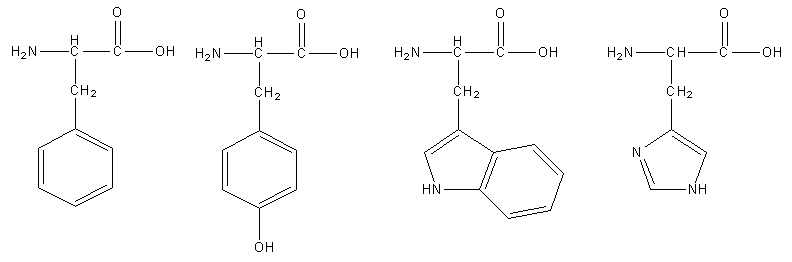
Phenylalanine is a precursor of phenethylamine. What do these aromatic amino acids do. There are four main aromatic amino acids and these four 4 amino acids have unique and different benefits and contributions. Among the 20 standard amino acids the following are aromatic.
Tyrosine and tryptophan absorb more than do phenylalanine. These are the ones that have aromatic groups in their molecule. For example based on the propensity of the side chain to be in contact with water amino acids can be classified as hydrophobic low propensity to be in contact with water polar and charged energetically favorable contact with water. Tryptophan is a precursor of the neurotransmitter serotonin.
The four main aromatic amino acids are phenylalanine tryptophan histidine and tyrosine and there are three 3 minor ones such as thyroxine 5 hydroxytryptophan and l dopa. Tryptophan is responsible for most of the absorbance of ultraviolet light ca.