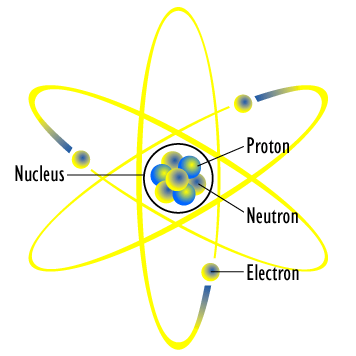
Bohor Model

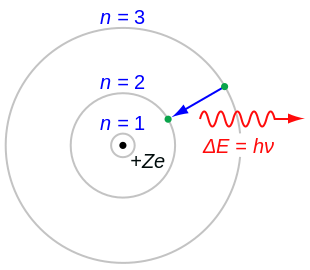
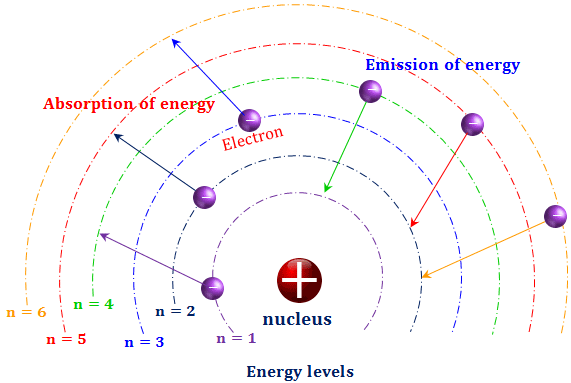
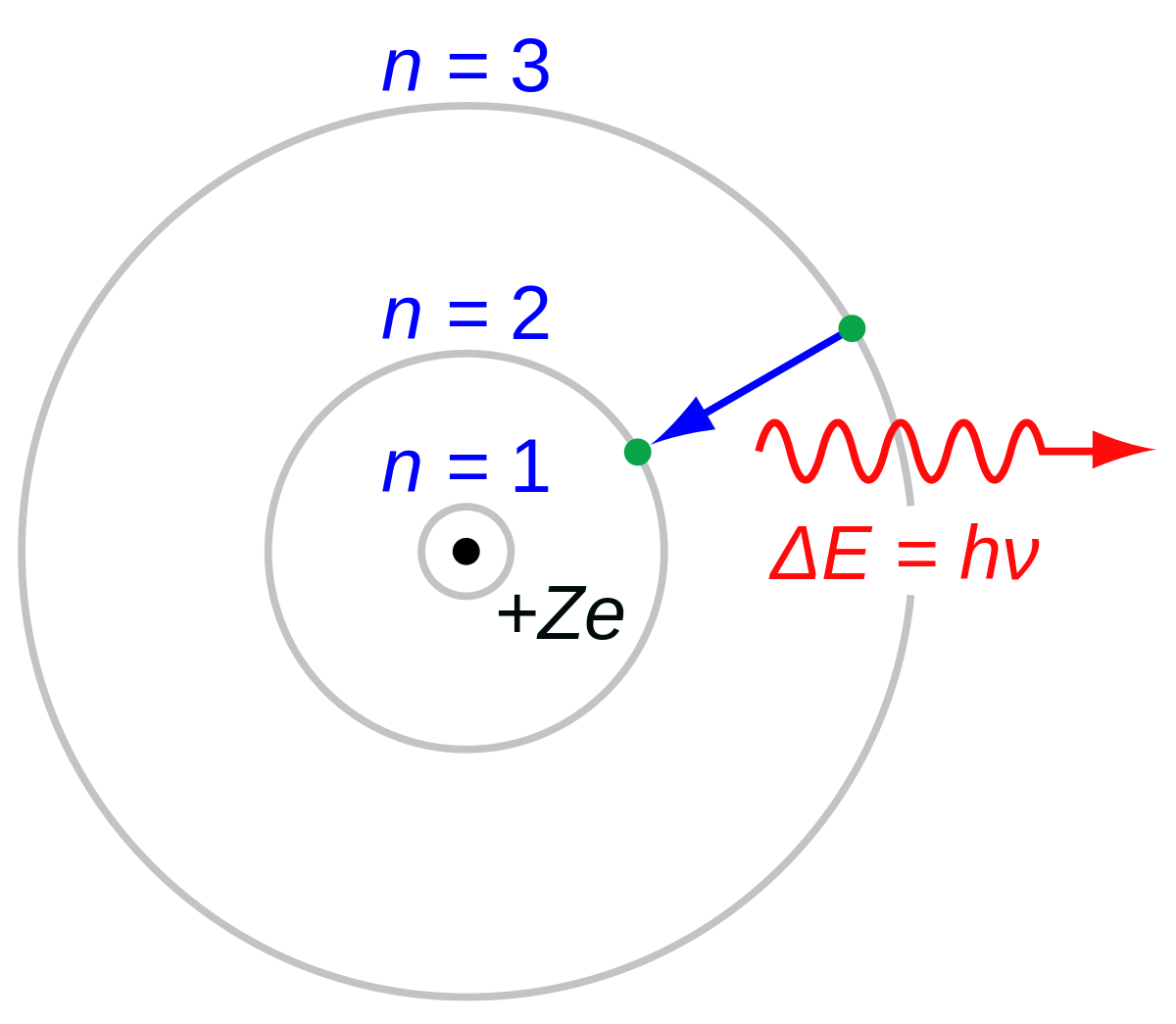
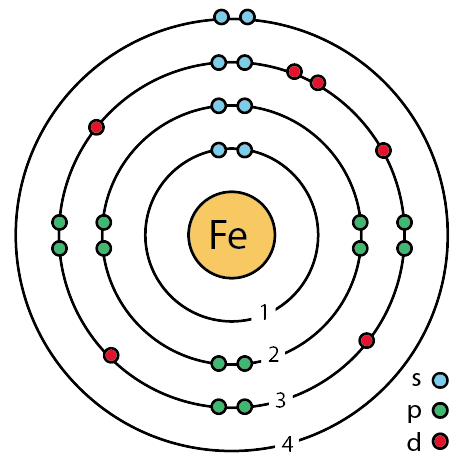
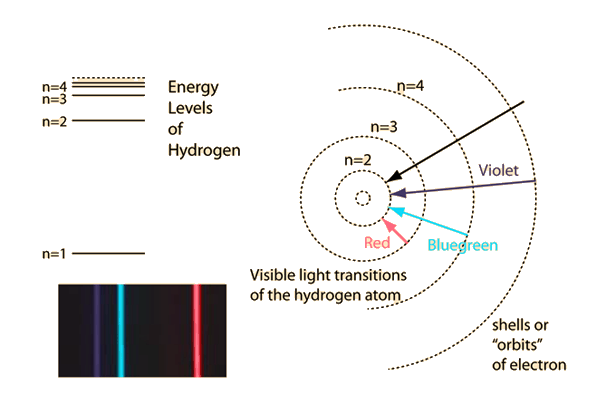
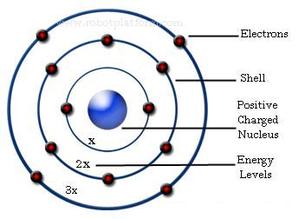
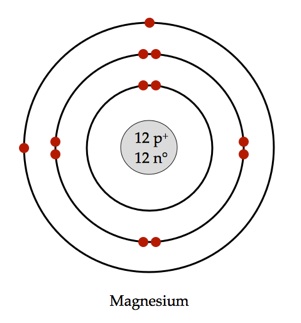
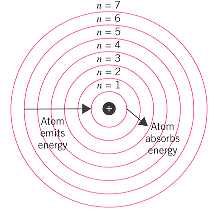
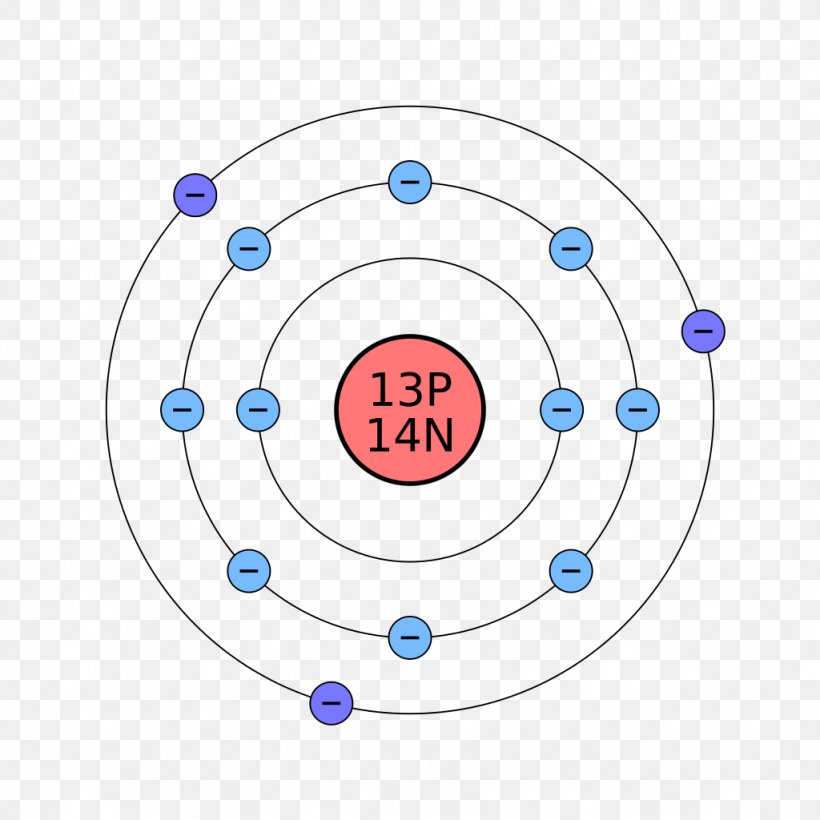
Bohr used the term energy levels or shells to describe these orbits of differing energy.
Bohor model. Overview of the bohr model niels bohrproposed the bohr model of the atom in 1915. The bohr model of the atom a radical departure from earlier classical descriptions was the first that incorporated quantum theory and was the predecessor of wholly quantum mechanical models. Here s a closer look at the bohr model which is sometimes called the rutherford bohr model. Although revolutionary at the time the bohr model is a relatively primitive model of the hydrogen atom compared to the valence shell atom.
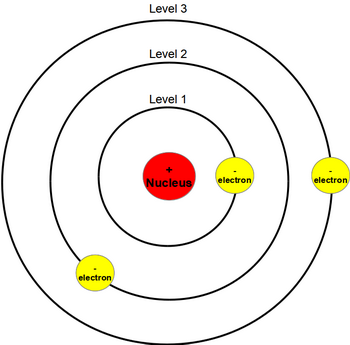
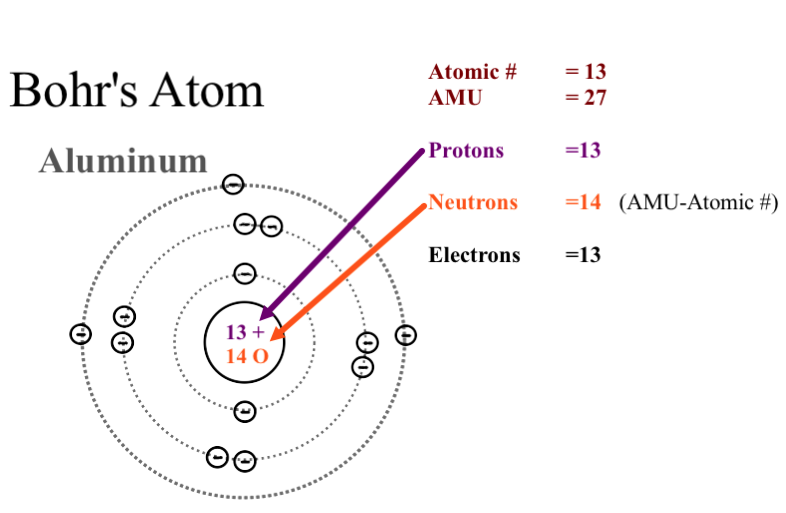
As an initial hypothesis it was derived as a first order approximation to describe the hydrogen atom. In atomic physics the bohr model or rutherford bohr model presented by niels bohr and ernest rutherford in 1913 is a system consisting of a small dense nucleus surrounded by orbiting electrons similar to the structure of the solar system but with attraction provided by electrostatic forces in place of gravity after the cubic model 1902 the plum pudding model 1904 the saturnian. Proposed by danish physicist niels bohr in 1913 this model depicts the atom as a small positively charged nucleus surrounded by electrons that travel in circular orbits defined by their energy.